MTS Helpdesk
Question:
What are the elements of the Analytical Procedure Lifecycle, as mentioned in guidance documents such as ICH Q14 and USP <1220>?
Answer:
An analytical procedure (or method) lifecycle consists of the activities associated with the procedure development, validation, routine use, transfer to another laboratory, and change control, throughout the period of time that it is in use.
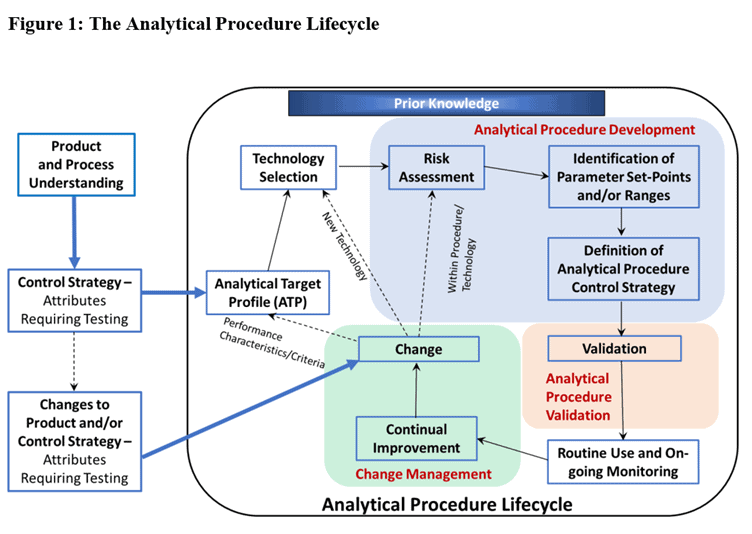
In section 2.2 of the ICH guideline Q14, Analytical Procedure Development, there is a figure which depicts the elements of the analytical procedure lifecycle. This diagram provides an excellent overview of the activities involved and it is described fully in the video below.
Submit a Question to the MTS Helpdesk
Do you have a question for our expert relating to the topic of analytical chemistry? Use our enquiry form to send it to us.
Related content from our Resources Library
Services related to this content that we offer
A full discussion of analytical procedure lifecycle management is included in our courses, ‘Validation, Verification and Transfer of Methods for Pharmaceutical Analysis‘, and ‘Validation, Verification and Transfer of Methods for Biopharmaceutical Analysis‘.
Visit our courses page for a full list of available courses and the schedule of currently available course dates.