Blog
Forced Degradation (1) HPLC (8) ICH (10) Lifecycle (5) Method development (2) Methods (1) MTS (1) MTS Helpdesk (11) OOS/OOT (1) Procedures (1) Stability (3) Troubleshooting (1) Validation (15) Verification (1) Video (5) Webinar (2)
-
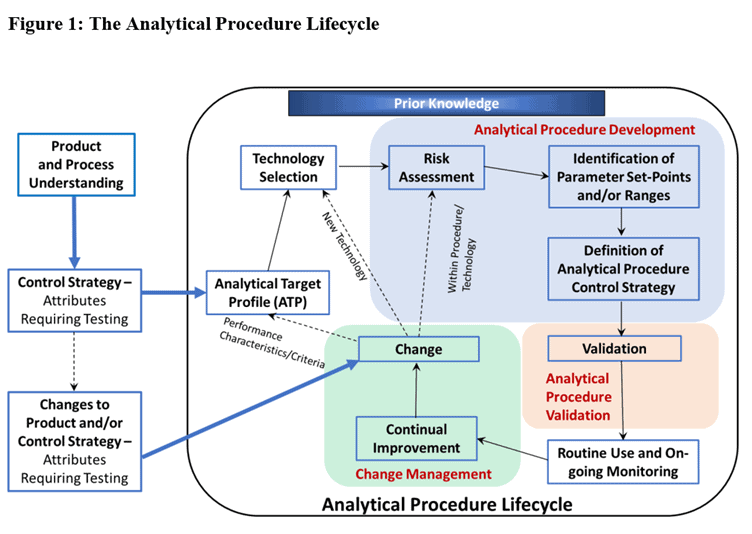
What is an Analytical Procedure Lifecycle?
MTS Helpdesk Question: What are the elements of the Analytical Procedure Lifecycle, as mentioned in guidance documents such as ICH Q14 and USP <1220>? Answer: An analytical procedure (or method) lifecycle consists of the activities associated with the procedure development, validation, routine use, transfer to another laboratory, and change control, throughout the period of time…
-
Self-Paced Online Training Option for HPLC Troubleshooting Course
Our course, ‘How to Troubleshoot HPLC’ is now available as a self-paced online training course. This means you can access the course over a period of 1 month in our virtual learning environment (e-MTS) in the form of on demand videos and exercises that you complete at a time to suit you. It’s perfect if…

-
New Course: What’s New in ICH Q2(R2) and Q14?
We are pleased to announce that we have a new short course on the topic of the ICH guidance documents, Q2(R2) and Q14, that is aimed at both our previous learners who have completed our validation course, and also at anyone who is experienced in validation and wants to know more. The course is currently…

-
ICH Q2(R2) & Q14 Now Available
The final versions of ICH Q2(R2) Validation of Analytical Procedures, and Q14 Analytical Procedure Development, are now available on the ICH website. You can view the pdfs using the buttons below. Related content from our Resources Library Services related to this content that we offer MTS offers a new short course relating to these updates,…
-
Updated ICH Q2(R2) and New Q14 Guidelines Finalised
The Assembly of the ICH met in-person in Prague on 31 October & 1 November 2023. The revised guideline on “Validation of Analytical Procedures”, ICH Q2(R2), and the new guideline on “Analytical Procedure Development”, ICH Q14, reached Step 4 and were adopted by the ICH Assembly Regulatory Members. The final versions of both guidelines are…

Forced Degradation (1) HPLC (8) ICH (10) Lifecycle (5) Method development (2) Methods (1) MTS (1) MTS Helpdesk (11) OOS/OOT (1) Procedures (1) Stability (3) Troubleshooting (1) Validation (15) Verification (1) Video (5) Webinar (2)
