MTS Helpdesk
Question:
Is the limit of quanitation always important when validating methods for impurities?
Answer:
Defined as “the lowest amount of analyte in a sample which can be quantitatively determined with suitable precision and accuracy” in ICH Q2, limit of quantitation (or ‘quantitation limit’ in the ICH terminology) is an important method performance characteristic which is typically demonstrated during analytical method validation.
Although the concept of limit of quantitation (LOQ) is relatively straightforward, it is often a source of confusion during method validation. This appears to be due to a number of factors:
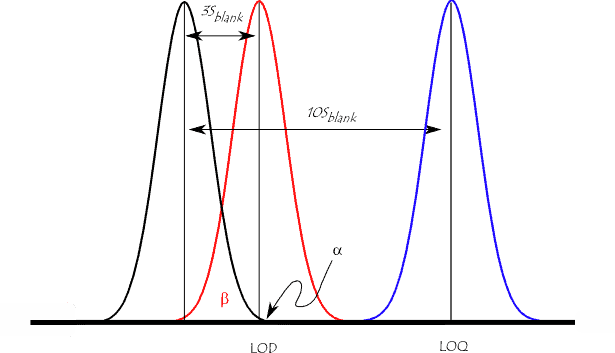
1. Determining the LOQ
Because the method validation guidance in ICH Q2 presents a number of methods for estimating the LOQ, it is tempting to conclude that it should be investigated during method validation studies. However, the determination of the LOQ is integral to the development of an analytical method which seeks to quantify low levels of compounds in sample matrices, e.g. the determination of impurities and/or degradation products. Therefore it should be established that the required level can be achieved when developing the method and then merely confirmed as part of method validation.
2. Suitable Precision and Accuracy
The LOQ is expressed in terms of ‘suitable precision and accuracy’, therefore it is essentially a user defined level since the analyst must decide what level of precision and accuracy is suitable. For example, a requirement for precision of less than or equal to 20 %RSD would result in a higher LOQ than a requirement of less than or equal to 25 %RSD.
3. Reporting Thresholds
For many methods the lowest concentration level that is required for a method is defined by the reporting threshold. For example, an impurities method may have a reporting threshold of 0.05% w/w with respect to the amount of drug present, as directed in ICH Q3A. Therefore, in method development it would be ensured that the method is designed to work at 0.05%, and in validation it would be demonstrated that suitable precision and accuracy are obtained at 0.05%. In effect, the actual LOQ for the method is not relevant since no value below the reporting threshold will be reported.
So, to go back to the original question, “Is the limit of quanitation always important when validating methods for impurities?”, the answer depends on the requirements for a particular method. If the true LOQ for the method is required then it does matter and should be thoroughly determined during method development and confirmed during method validation. However, if the lower limit of the range of the method is predetermined, such as a reporting threshold, then whether the method is actually capable of quantitative measurement below this value is not relevant, thus it may not matter (provided of course that the method is capable of quantitative measurement at the reporting threshold).
Submit a Question to the MTS Helpdesk
Do you have a question for our expert relating to the topic of analytical chemistry? Use our enquiry form to send it to us.
Related content from our Resources Library
Services related to this content that we offer
A full discussion of the role of the LOQ is included in our courses on analytical method validation, ‘Validation, Verification and Transfer of Methods for Pharmaceutical Analysis‘, and ‘Validation, Verification and Transfer of Methods for Biopharmaceutical Analysis‘.
Visit our courses page for a full list of available courses and the schedule of currently available course dates.