MTS Helpdesk
Question:
How long does it take to equilibrate a column on an HPLC system before the first injection can be performed?
Answer:
This is a question commonly asked by HPLC analysts, particularly those relatively new to the technique. The range of currently available column dimensions means that a ‘one size fits all’ approach is not appropriate. The amount of mobile phase which should be flushed through a column before it is ready to use is usually expressed in terms of the column volume, the amount of mobile phase required to fill the column.
When a column has just been installed on a reversed phase HPLC system then it will typically require between 10 and 20 column volumes before it is fully equilibrated and ready to use. However, some applications are likely to require additional column volumes. Examples are methods which include ion-pairing reagents and chiral HPLC methods. In these cases suitable equilibration should be investigated and documented for future use. A starting point for investigation may be approximately 30 column volumes. When re-equilibrating after a gradient injection has been run (prior to the next injection) 10 column volumes is recommended.

Although we refer to the column volume, the volume that we are interested in is more correctly called the void volume, Vm. This is the volume of the HPLC column that is not taken up by the stationary phase. This is typically approximately 70% of the total column volume. There are two methods that you can use to calculate the void volume.
Method 1
Use the dimensions of the column and the formula for the volume of a cylinder.
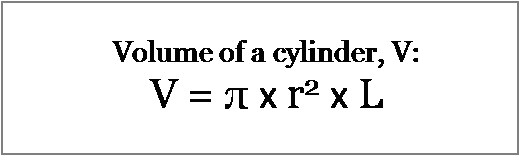
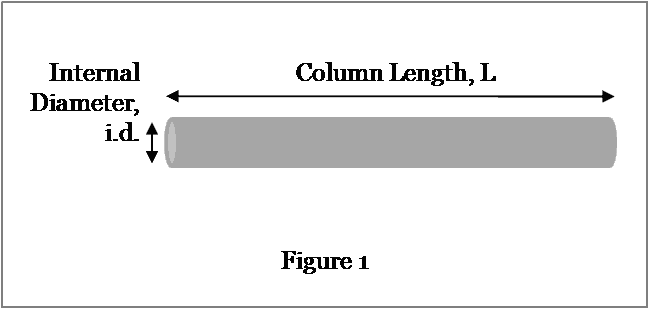
The dimensions of interest on a HPLC column are shown in Figure 1 below.
The result of the calculation above is the total column volume. To convert this value to the void volume we multiply by 70%, therefore the formula becomes:

The radius, r, is equal to the internal diameter divided by two:
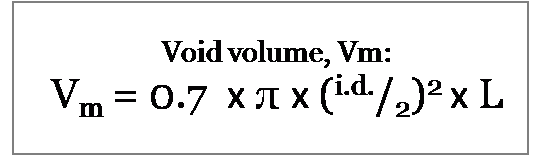
Example
A HPLC column of internal diameter 4.6mm and length 10cm:
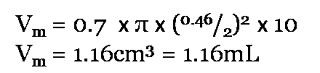
Note: express both i.d. and length in cm.
To calculate the required equilibration time simply multiply the calculated void volume (in mL) by the number of required column volumes, e.g. 10, then divide by the flow rate (in mL/min)to determine the total time required.
For a flow rate of 1 mL/min on the column above, this would result in an equilibration time of 11.6 minutes for 10 column volumes.
Method 2
Inject an unretained solute to obtain t0, the column dead time (minutes). Then multiply this by the flow rate to obtain the void volume:
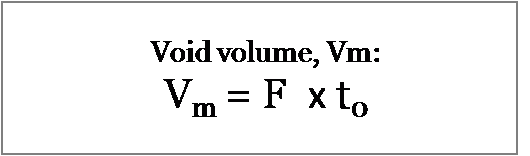
Where F is the flow rate expressed in mL/min.
Example
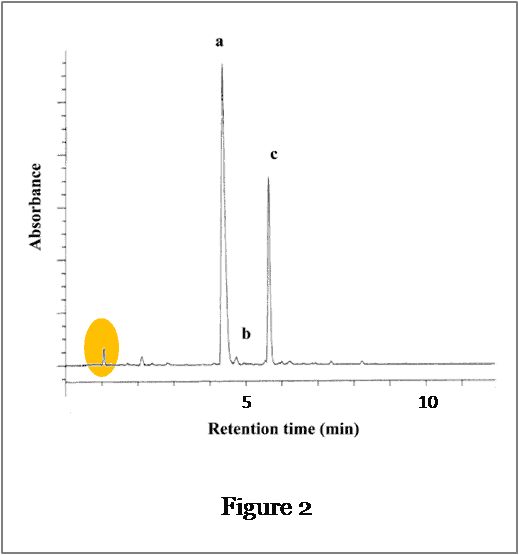
The flow rate for the method is 1.0 mL/min.
The column dead time, t0, is obtained from the chromatogram in Figure 2 and is equal to 1.05 minutes.
Therefore, Vm = 1.0 x 1.05 = 1.05mL
As previously, to calculate the required equilibration time you can multiply the calculated void volume (in mL) by the number of required column volumes, e.g. 10, then divide by the flow rate (in mL/min) to determine the total time required.
However, you can see that we multiplied by the flow rate and then divided by the flow rate. The purpose of this was to show the principle of the calculation and compare to method 1.
In fact, the required equilibration time is just t0 multiplied by the number of column volumes required. In this example, the equilibration time is simply 1.05 x 10 = 10.5 minutes.
The MTS HPLC calculator will perform all of these calculations for you. You can request a copy of the calculator on our Resources page.
Submit a Question to the MTS Helpdesk
Do you have a question for our expert relating to the topic of analytical chemistry? Use our enquiry form to send it to us.
Related content from our Resources Library
Services related to this content that we offer
We have a number of training courses on the topic of HPLC, which includes an introductory course, and courses on HPLC method development (including a course specifically aimed at developing stability indicating methods) and HPLC troubleshooting.
Visit our courses page for a full list of available courses and the schedule of currently available course dates.
If you are looking for a book on HPLC, our ‘Introduction to HPLC for Pharmaceutical Analysis‘ by Oona McPolin, may be just what you need.

